How is protein tested with a nitrogen and amino acid analysis?
With a measurement of the nitrogen content in your protein powder shake there are two methods that can be used; Kjeldahl and Dumas methods. In both, the nitrogen from the amino acids released can be used to calculate the protein content. They each have their advantages and disadvantages in the measurement of whey products. However these methods both have the disadvantage that seperate free amino acids or nitrogenous products can bring the protein content higher. This is because every amino acids contains nitrogen that can't be distinguished in this way. Then there is the question of whether there has been measured on crude protein or dry matter.
Amino acid analysis on protein
A different analysis, the amino acid analysis, always comes in handy because of this reason. This is the only way to exclude artificial addition of nitrogen-containing substances such as separate amino acids, creatine, and collagen. Any type of whey protein contains a specific profile of its amino acids. For this reason, an addition of single amino acids or a product made up of amino acids will always be detected. We also can then see if a cheaper protein product has been added.
A better method for distinguishing between individual amino acids is a measurement of non-bound amino acids. This way there is no doubt when free amino acids are added.
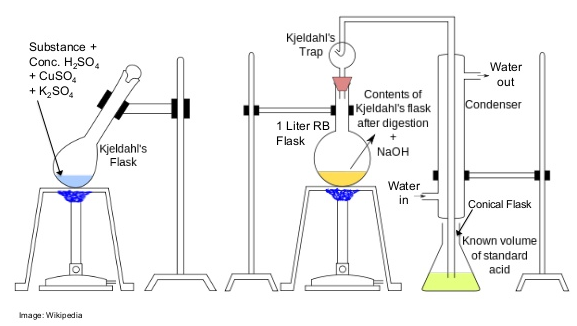
Kjeldahl method (nitrogen analysis)
With this method sulfuric acid is first added to decompose the organic compounds (amino acids). Then sodium hydroxide will be added (lye) to allow the nitrogen that is released to be converted into ammonia. The released ammonia is mixed with a boric acid solution and then hydrochloric acid is added to it. With different measurements during the process the nitrogen content can be calculated. Then the crude protein content of milk products such as whey is calculated by multiplying by a factor of 6.38.
The whole process takes a couple of hours with a lot of chemical waste which is not too pleasant to work with. But it's the cheapest and most accurate way currently. Nowadays there are also Kjeldahl analysis devices that operate almost automatically which saves a lot of time and increases safety.
Automatic kjeldahl analyzer

Dumas method (nitrogen analysis)
This analysis takes place fully automatically by means of an Dumas analysis device. The device uses heat to decompose the nitrogen compounds forum your protein powder. First, the whey is incinerated at 1100ºC and the released nitrogen compounds are converted to nitric oxide (NO2) at 750ºC. The water vapor is removed and the NO2 is converted in the final stage to dinitrogen (N2) at 600ºC. Then the gas will be purified and measured by means of a gas chromatograph.
This process takes about four minutes. The problem of this method is the price of the device and its maintenance. The cost will be much higher given that some parts need to be replaced regularly, also the machine has to be cleaned after each analysis. There could also be a possible greater risk of deviating measurements. For these reasons the Kjeldahl method is prefered with the measurement of protein content in whey products.

Amino acid analysis explained
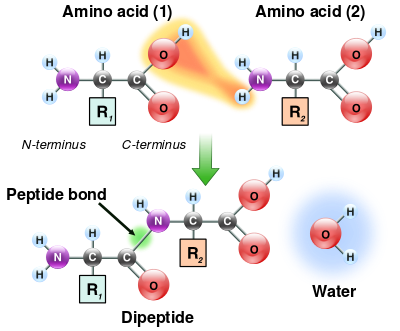
For this a mass spectrometer is used to 'measure' each amino acid separately. This is done by comparing the found substances (amino acids) with a database of known substances. By means of chomatography all substances are first separated until only the protein remains. Whey protein, like any protein, consists of peptides. These are different amino acids linked together. And each peptide is in turn linked to each other to form a protein.
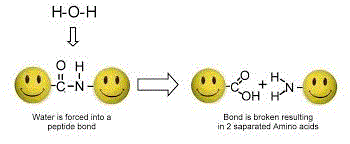
By means of hydrolysis the peptides are first separated from each other, and finally the amino acids by further hydrolysis. The mass of each amino acid can be detected by the mass spectrometer and is then compared with the database. Afterwards each amino acid will be counted and by adding up all the amino acids you'll find the total protein content. This method is of course the most expensive and the most time-consuming. But it is the only way to detect 'amino spiking' with loose amino acids or the addition of other nitrogenous substances.
Free amino acid analysis
Also all non-bonded (free) amino acids can be measured in this way to detect amino spiking in your protein powder shake. Hydrolysis is not used in this measurement so that only the already existing individual amino acids will be measured. But added creatine or collagen are still indistinguishable from whey protein then because the amino acids of these substances are bound.
Mass spectrometer:

Sample analysis
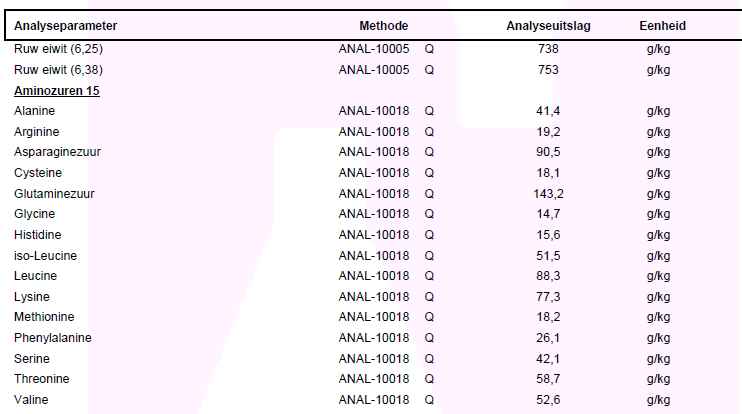
Below is an example of a Kjeldahl nitrogen analysis and a amino acid analysis by 15 amino acids. Why not 18 can be read here. The correct calculation of the nitrogen analysis of whey protein is Ruw eiwit/Crude protein (6.38). The values are in grams per kilogram. By putting a comma before the last digit this becomes the percentage. 75.3% for crude protein in this example.
A deviation of maximum 7% (crude protein) as compared to the value on the label is within the boundries. For example: If there has been declared 80% on the label -> ((75,3 - 80) / 80) x 100 = -5,9%, so within the allowed maximal deviation.
Relevant lecture:

